
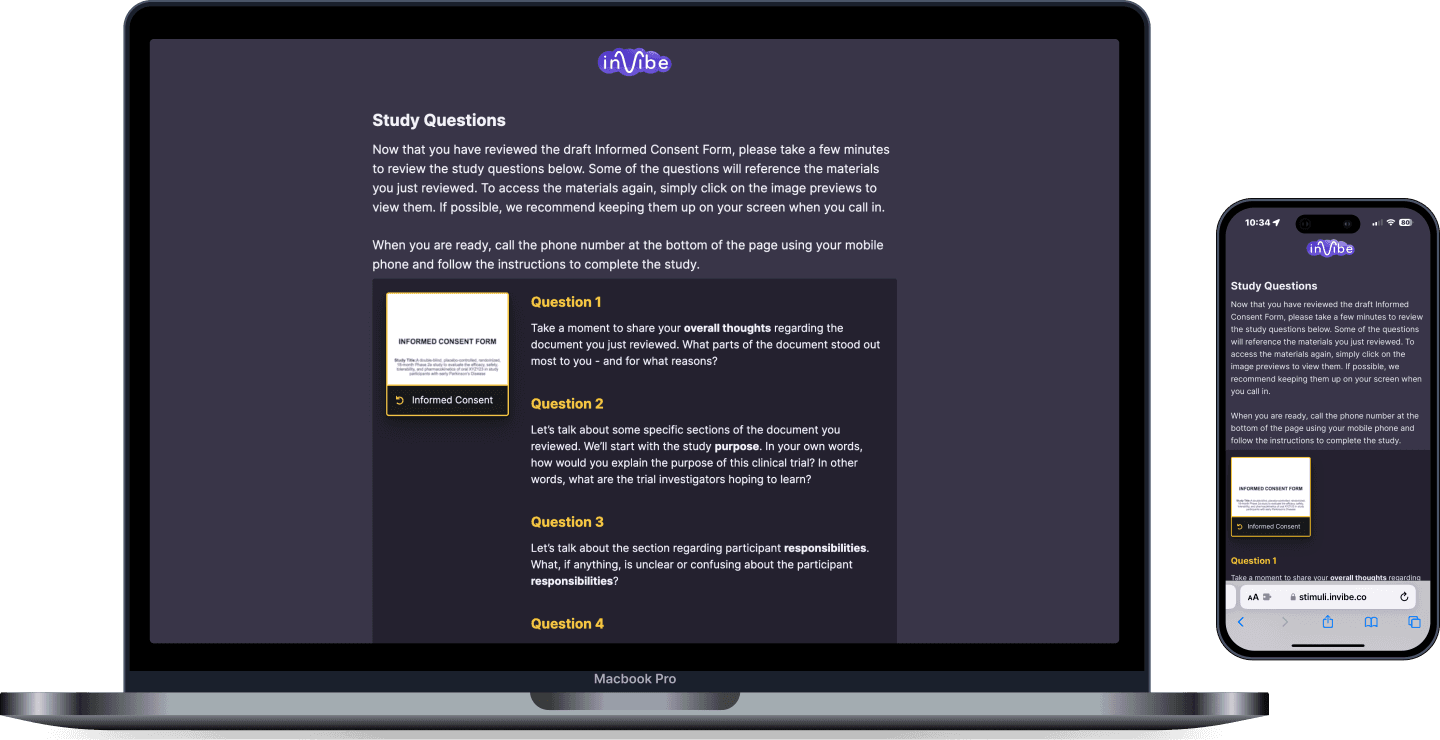
Listening to the Voice of the Patient

Obtain patient provided feedback via their voice response on your protocol, recruitment strategy, consent document, and retention solutions
Powered by Technology, Simplified with AI, Interpreted By Our Linguistic Experts
Study sponsors and CROs understand the vital importance of listening to patients and caregivers throughout the drug development lifecycle. In fact, the FDA’s Patient Focused Drug Development (PFDD) guidance is encouraging our industry to take a more patient-centric approach — as listening is essential for designing truly patient-centered studies.
However, listening to patients has not always been easy to accomplish. Traditional methodologies tend to be somewhat burdensome, time-consuming, and biased. inVibe has solved those challenges with a methodology and technology to collect and analyze real patient voices that are simple, systematic, and scalable across the organization.
Listening Is Core to Our Mission
inVibe is a key component of how THREAD is helping to modernize the collection of data, reduce barriers to participation, and enable patients and their caregivers to have a true voice in how they will experience clinical trials.
Study sponsors and CROs leverage inVibe both pre-launch (to optimize study design and educational materials), as well as during active studies (to diagnose issues causing slow recruitment or poor retention). Specific use cases include but are not limited to, understanding disease burden, protocol design, and study burden — as well as evaluating study materials and recruitment assets.

How It Works
inVibe is templated and customizable, patient-centric, technology-driven market research.
- inVibe quickly recruits patients similar to the study’s inclusion/exclusion criteria — and invites them to participate in a simple automated voice interview (no longer than 30 minutes of a patient’s time).
- inVibe technology then captures the patient’s voices to truly bring the emotions to life. Combining machine learning algorithms, GPT-4, and human analysis, the voice is analyzed so that sponsors can understand the WHY behind the WHAT.
- Within 3 weeks, sponsors receive crucial patient-generated evidence to inform study design and/or actionable recommendations to fix study challenges. Results are delivered in an easy-to-digest interactive dashboard with summary reports — that not only provides clear assessments but translates them into implications. This tailored approach to patient voice collection ultimately results in:
- Fewer protocol amendments
- Faster recruitment / Improved retention
- Better & more personalized medicines for patients
…I also really like this system of collecting data, talking to this automated system and just getting to freely talk so that I don’t have to really do any typing or work involved. I just get to talk and think freely. There’s no one on the other end of the phone judging my response. So, actually, I like this system. So if you could do that, that’d be good.




















